


projects.
2 Neuregulin and Schwann cell development
3 Kif1b and mbp RNA localization
4 Inflammation and microglia development
5 Oligodendrocyte specification and migration
6 Microglia and Cell Biology of Phagocytosis






Gpr126
and myelination
Starting with the analysis of two mutants (st49 and st63) from our genetic screen
(Pogoda et al. 2006, Developmental Biology, 298: 118-131), we discovered that
the adhesion G protein coupled receptor Gpr126 is required for Schwann cells to
initiate myelination (Monk et al. 2009, Science 325: 1402-1405). In gpr126 mutants,
Schwann cells arrest just before the onset of myelination. Elevation of cAMP restores
myelination in gpr126 mutants. After the early signaling defect is bypassed by
transient elevation of cAMP, Schwann cells in gpr126 mutants can form a mature
myelin sheath, and maintain it for months (Glenn and Talbot, 2013, Development
140: 3167-3175). Thus our analysis indicates that Gpr126 signaling is required
specifically at the onset of myelination.
The function of Gpr126 in myelination is widely conserved among vertebrates, as
we showed by analyzing Gpr126 mutant mice (Monk et al. 2011, Development
138: 2673-2680) and, as part of a collaborative study, mutations in GPR126 in
human patients (Ravenscroft et al. 2015, American Journal of Human Genetics).
Current experiments are focused on understanding the Gpr126 signaling pathway
and its interaction other factors that regulate Schwann cell development and
myelination. We are working to determine if type IV collagen, a component of the
Schwann cell extracellular matrix that can bind and activate Gpr126 in cultured
cells (Paavola et al. 2014 Science Signaling 7: ra76), is important for Gpr126
signaling and myelination in vivo. In addition, we are investigating how Gpr126
activity intersects with Neuregulin signaling to control Schwann cell myelination.
Neuregulin and Schwann
cell development
Through mutational analysis in zebrafish, we discovered that ErbB receptor tyrosine
kinases are essential for directed migration of Schwann cells in growing nerves
(Lyons et al. 2005, Current Biology 15: 513-524). Previous work in mammals has
indicated that ErbB receptors and their Neuregulin ligands control many aspects
of Schwann cell development, including proliferation and myelination. We found
that zebrafish erbb mutants have defects in Schwann cell migration, in addition to
proliferation and myelination. By applying a drug that blocks ErbB signaling after the
start of migration, we showed that ErbB signaling is required for directed migration
of Schwann cells, not simply for their motility. In addition, we found that ErbB
signaling is required for Schwann cells to extend processes into bundles of axons
during radial sorting, the process that pairs Schwann cells with the axons segments
they will myelinate (Raphael et al. 2005, Glia 59: 1047-1055).
Our mutational studies and other experiments provide evidence that Neuregulin
(Nrg) type III signals are essential guidance cues for migrating Schwann cells
(Perlin et al. 2011, Development 138: 4639-4648). By controlling Schwann cell
proliferation and migration, Nrg1-type III ensures that Schwann cells are present
in the proper numbers and proper places to myelinate the appropriate axonal
segments. Our current experiments are focused on understanding how Nrg-ErbB
signaling is regulated to direct Schwann cell migration and on analyzing the
functions of other Neuregulin isoforms. back to top
Kif1b and mbp
RNA localization
In the CNS of mammals and zebrafish, mRNAs encoding myelin basic proteins
(Mbp) and a small number of other structural components of myelin are localized to
processes of myelinating oligodendrocytes. Until recently, the role of specific myelin
mRNA localization in oligodendrocyte differentiation was not understood. We have
discovered that mutation of kif1b, which encodes a kinesin motor, causes defects
in mbp mRNA localization in oligodendrocytes (Lyons et al. 2009, Nature Genetics
41: 854-858). In kif1b mutants, mbp mRNA is present in oligodendrocyte cell
bodies, but not in myelinating processes. Mbp protein is present in the processes
of mutant oligodendrocytes despite the lack of mbp mRNA. We are working to
determine if the Kif1b motor acts directly to transport mRNA cargo into myelinating
processes.
The finding that mbp mRNA is mislocalized in kif1b mutants provided the opportunity
to investigate the role of mRNA localization in oligodendrocytes. In kif1b mutants,
long stretches of compact myelin-like membranes were observed surrounding
neuronal cell bodies and in processes that did not ensheath axons. Mbp protein is
present at abnormally high levels in oligodendrocyte cell bodies of kif1b mutants,
suggesting that mislocalized myelin proteins contribute to aberrant membrane
compaction in kif1b mutants. According to this model, the role of mRNA localization
in oligodendrocytes is to restrict the localization of Mbp and thereby prevent the
deleterious action of myelin proteins in other parts of the cell.
In ongoing experiments, we have defined a region on the 3’ UTR of mbp mRNA
that is sufficient to localize a heterologous mRNA to myelinating processes. We
are using genome editing methods to delete this localization element and other
sequences to define regions of the mbp mRNA that are required for its localization
to myelin. back to top
Inflammation and
microglia development
Through mutational analysis in zebrafish, we discovered that the noncanonical NOD
-like receptor (NLR) Nlrc3-like is essential for formation of microglia (Shiau et al.,
2013, Cell Reports 5: 1342-1352). Although most NLRs trigger inflammatory
signaling, we found that Nlrc3-like acts cell autonomously in microglia precursor
cells to suppress unwarranted inflammation in the absence of infection. In nlrc3-
like mutants, primitive macrophages initiate a systemic inflammatory response with
increased proinflammatory cytokines and actively aggregate instead of migrating into
the brain to form microglia. In addition, neutrophils infiltrate the brain in nlrc3-like
mutants, whereas these immune cells are excluded from the CNS of wildtype
siblings. Together, these results demonstrate that Nlrc3-like prevents inappropriate
macrophage activation, thereby allowing normal microglial development. Our results
support the model that Nlrc3-like may regulate the inflammasome and other
inflammatory pathways is to prevent the initiation of inappropriate inflammatory
responses. Mechanistic investigation of Nlrc3-like will advance the understanding
of inflammatory signaling in normal development and disease. back to top
Oligodendrocyte
specification and migration
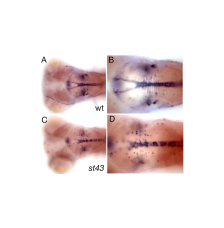
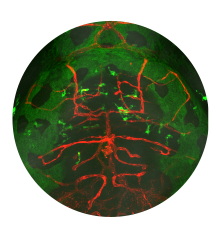
In a genetic screen for mutations that disrupt myelination, we identified a gene encoding a novel zinc finger (ZNF) protein that regulates myelin development in the brain and spinal cord. znfst78 mutants have reduced mbp at 5 dpf, but mbp is expressed at later stages and some mutants survive to adulthood. The primary defect in mutants is that oligodendrocyte precursor cells (OPC) fail to migrate out of the ventral neural tube into the parenchyma of the CNS. Tissue-specific rescue experiments indicate that the protein functions autonomously in oligodendrocytes. notch3, which is also involved in specification of OPCs (Zaucker et al. 2013, Disease Models and Mechanisms 6: 1246-1259), is apparently required in a distinct population of OPCs; this suggests that different genes specify different populations of zebrafish OPCs, as has been shown previously in mammals. We are currently investigating the target genes that znf regulates to promote OPC migration. back to top
Microglia and Cell Biology of
Phagocytosis
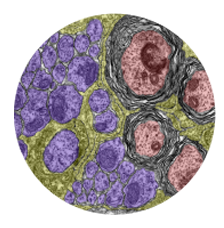
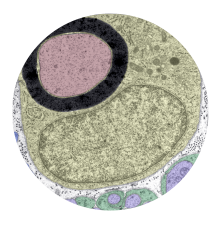
Microglia are resident macrophages of the CNS that are essential for phagocytosis of apoptotic neurons and weak synapses during development. Using forward and reverse genetic approaches, we discovered that RagA and Lamtor4, two components of the Rag-Ragulator complex, are essential regulators of lysosomes in microglia (Shen et al. 2016, Cell Reports 14: 547-559). In zebrafish lacking RagA function, microglia exhibit an expanded lysosomal compartment, but they are unable to properly digest apoptotic neuronal debris. Our analysis reveals an essential role of the Rag-Ragulator complex in proper lysosome function and phagocytic flux in microglia. We are currently working to define the mechanisms by which the Rag-Ragulator complex regulates lysosomes, and to define the function of RagA in other cell types.
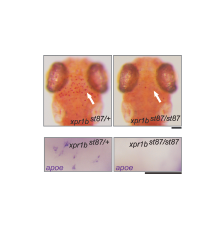
In a genetic screen, we uncovered an essential role for the phosphate exporter xpr1b in microglia
and other tissue-resident macrophages (Meireles et al. 2014, Cell Reports 6: 1659-1667). xpr1b mutants lack microglia and display an osteopetrotic phenotype characteristic of defects in osteoclast function. Our genetic analysis in zebrafish provides strong evidence that Xpr1b acts cell autonomously in the macrophage precursor population and has a unique and specific function in specialized tissue macrophages, including microglia and osteoclasts. Our hypothesis is that these phagocytic cells require Xpr1b to prevent harmful levels of phosphate from accumulating as they engulf apoptotic neurons, bone, and other debris rich in

talbot lab. stanford school of medicine. copyright © 2015. all rights reserved.